GLP-1 Therapy Part 1: Setting Realistic Weight Loss Expectations
I recently had the pleasure of speaking at the Colorado Academy of Nutrition and Dietetics Annual Conference on the topic of nutrition strategies for effective GLP-1 use in obesity treatment.
This premier event, dedicated to supporting nutrition and dietetics professionals in Colorado, offered a valuable opportunity to share updated evidence on GLP-1 therapies, while also debunking common myths to promote informed and unbiased nutrition counseling.
In this four-part series, I’m excited to share my key recommendations to help support your practice. In Part 1, we’ll explore key considerations for identifying appropriate GLP-1 candidates and highlight the importance of setting realistic weight loss expectations.
New to GLP-1 Therapy? Start here.
How GLP-1 Medications Work
Before starting a patient on a GLP-1 medication, it can be helpful explain how the therapy works and set clear expectations.
Reviewing the mechanism of action—specifically how it influences hunger, fullness, satisfaction, and food cravings—can help patients understand that weight loss results from a medication-induced calorie deficit, not from “burning fat” or “boosting metabolism”.
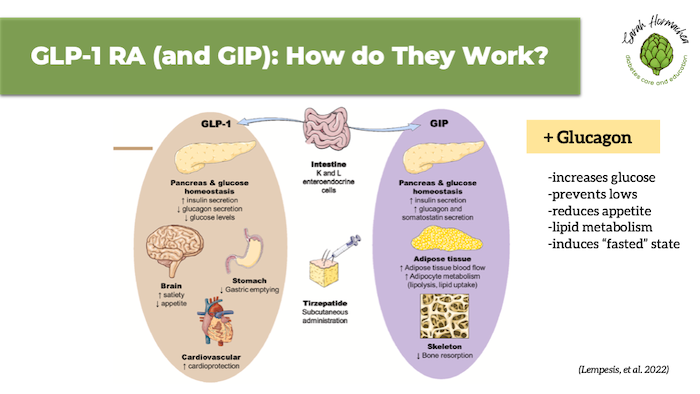
The image below highlights the mechanisms of both GLP-1 and GIP, along with a callout to the newest treatment option—a triple incretin that also includes glucagon—expected to be approved in late 2025 to early 2026.
When to Start—and When Not To
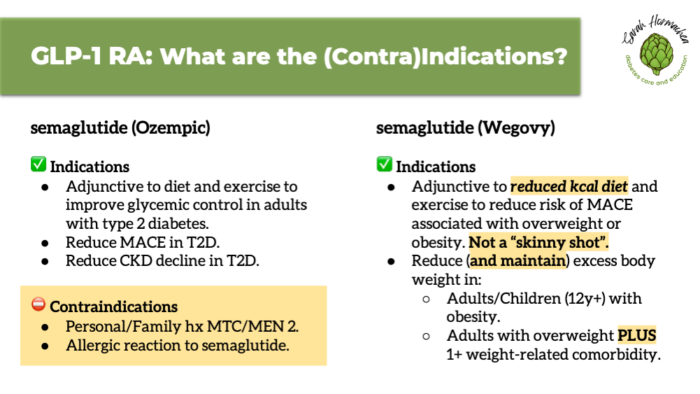
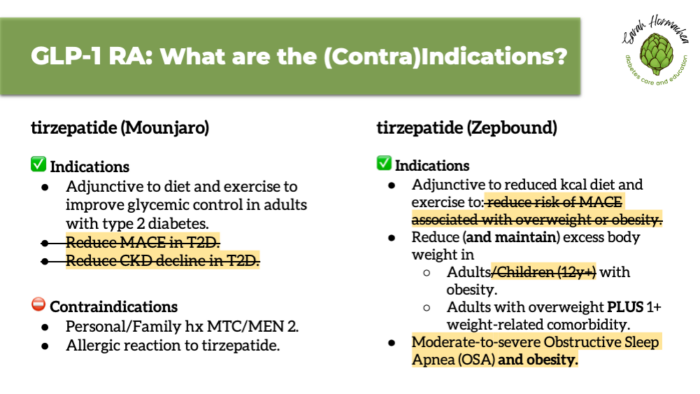
According to the package insert, GLP-1 therapy for obesity is indicated for individuals with a BMI ≥27 with a weight-related condition, or a BMI ≥30 alone.
It is intended to be used as adjunctive therapy—in addition to nutrition support or health coaching from a qualified professional. If not already doing so, individuals should be prepared to make lifestyle changes, including adopting a reduced-calorie eating pattern and increasing physical activity. It’s important for patients to understand that GLP-1 medications are not a “skinny shot,” but a tool designed to support metabolic health through long-term weight management.
Contraindications include a personal or family history of medullary thyroid carcinoma (MTC), multiple endocrine neoplasia syndrome type 2 (MEN 2), or a known allergic reaction to the medication.
There are also warnings and precautions which, while they may be valid reasons to avoid using a GLP-1 medication, are not strict contraindications. Understanding this distinction helps prevent misinformation and supports informed consent.
It’s also important to screen for any history of eating disorders, as the appetite-suppressing effects of GLP-1s may pose risks for certain individuals. Early and honest conversations like these promote safety, trust, and shared decision-making from the outset of treatment.

Realistic and Sustainable Weight Loss Goals
Setting safe and sustainable weight loss goals is essential for long-term success. Most experts agree that 0.5 to 2.0 pounds per week, or roughly 1% of body weight, is appropriate.
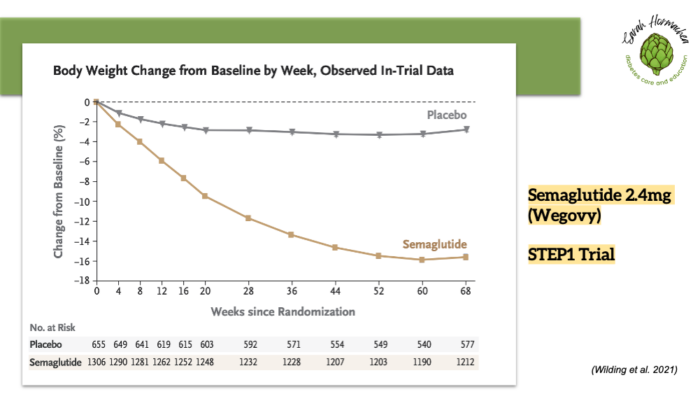
Semaglutide (Wegovy) and tirzepatide (Zepbound) have demonstrated significant average weight loss—14.9% and 20.9% of body weight at approximately one year, respectively.
While these outcomes are based on large clinical trials (STEP 1 and SURMOUNT-1), weight loss should still be approached with realistic expectations, personalized goals, and ongoing support.
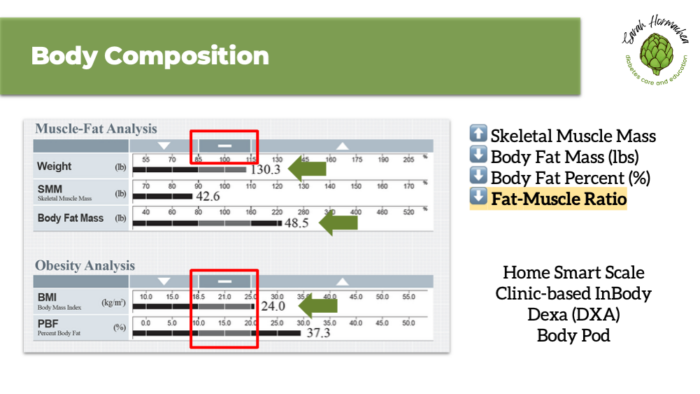
More important than net weight loss are changes in body composition—specifically, reducing body fat while minimizing the loss of lean muscle mass. Consider evaluating body composition using tools such as an InBody scale or DEXA scan to establish baseline values and monitor changes over time. This approach is especially valuable as we continue to refine diagnostic criteria for clinical obesity.

Key Takeaways
GLP-1 medications are powerful tools for obesity management, but they are most effective when paired with nutrition counseling and sustainable lifestyle changes. These medications support metabolic health by regulating appetite to create a calorie deficit, leading to weight loss.
Before starting therapy, it’s essential to assess a patient’s readiness for behavior change and review both contraindications and precautions to ensure safe and appropriate use.
While clinical trials show significant average weight loss with GLP-1 therapy, setting realistic, individualized goals remains key. Most individuals can expect to lose 0.5 to 2 pounds per week in a way that is both safe and sustainable. It’s equally important to focus not just on weight loss, but on preserving lean muscle mass throughout the process. Tools like InBody or DEXA scans can be helpful for monitoring body composition and supporting long-term health outcomes.
Stay tuned for Part 2 next week, where we’ll dive into managing and preventing side effects of GLP-1 therapy.
- Wilding JPH, Batterham RL, Calanna S, et al. Once-weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989-1002. doi:10.1056/NEJMoa2032183
- Lempesis IG, Liu J, Dalamaga M. The catcher in the gut: Tirzepatide, a dual incretin analog for the treatment of type 2 diabetes mellitus and obesity. Metabolism Open. 2022;16:100220. doi:10.1016/j.metop.2022.10022
- Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022; 387(3): 205-216.
- Almandoz JP, Wadden TA, Tewksbury C, et al. Nutritional considerations with antiobesity medications. Obesity. 2024;32(9):1613-1631. doi:10.1002/oby.24067
- CDC. Steps for losing weight. Healthy Weight and Growth. February 26, 2024. Accessed October 16, 2024. https://www.cdc.gov/healthy-weight-growth/losing-weight/index.html
- Rubino F, Cummings DE, Eckel RH, et al. Definition and diagnostic criteria of clinical obesity [published correction appears in Lancet Diabetes Endocrinol. 2025 Mar;13(3):e6. doi: 10.1016/S2213-8587(25)00006-3.]. Lancet Diabetes Endocrinol. 2025;13(3):221-262. doi:10.1016/S2213-8587(24)00316-4

You May Also Like

Diagnosing Type 1 Diabetes in Adults
August 10, 2022
Entrepreneurship in Diabetes Care and Education
November 26, 2024