Your Guide to Diabetes Supplies: Continuous Glucose Monitoring
Managing diabetes can be incredibly challenging, especially with the demands of traditional fingerstick glucose monitoring. Thankfully, advancements in technology and expanded insurance coverage have made continuous glucose monitors (CGMs) more accessible than ever. In this post, we’ll delve into what CGMs are, how they work, their benefits, and how they can enhance the quality of life for individuals managing diabetes.
All About Continuous Glucose Monitors
What is a Continuous Glucose Monitor?
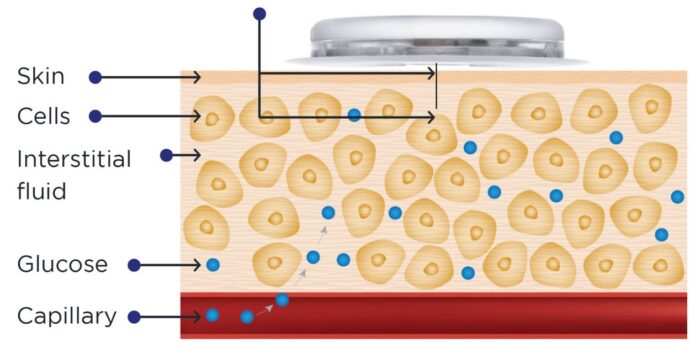
A continuous glucose monitor (CGM) is a small device that measures glucose levels in a person’s interstitial fluid and transmits this data to a receiver or smartphone. Primarily used by individuals with diabetes, CGMs provide real-time glucose readings, empowering users to make informed decisions about food, exercise, and medication adjustments.
In addition to offering real-time insights, CGMs can alert users when glucose levels are too high or too low. These alerts can be life-saving, particularly during sleep, when dangerously low blood sugar levels could lead to seizures or unconsciousness.
How do Continuous Glucose Monitors Work?
Continuous glucose monitors work by inserting a small sensor under the skin that measures glucose levels in real time. The sensor connects to a transmitter, which wirelessly sends the data to a device such as a smartphone, insulin pump, or dedicated receiver. These devices display glucose readings and provide alerts for high or low levels.
Unlike traditional fingerstick methods, CGMs measure glucose levels in the interstitial fluid—the fluid between cells—rather than directly in the blood. While interstitial glucose levels closely reflect blood glucose, there can be a delay of up to 15 minutes in the readings.
Are Continuous Glucose Monitors Accurate?
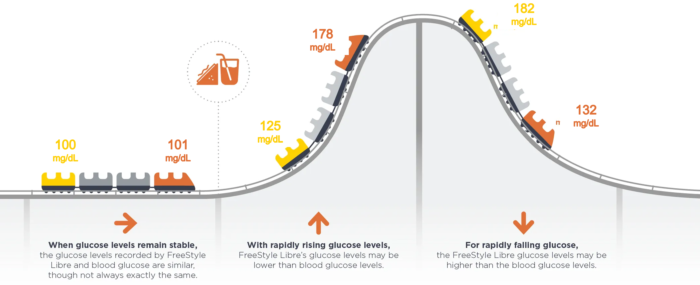
Continuous glucose monitors are generally accurate, though not 100% foolproof. Accuracy largely depends on the type of device, calibration process, individual physiology and certain environmental factors.
Continuous glucose manufactures provide guidance on how to interpret and improve sensor accuracy. Both Dexcom G6 and G7 are factory calibrated for optimal accuracy at 100 mg/dL (plus or minus 20%). When a glucometer reads 100 mg/dL, a Dexcom sensor is a close match if it’s between 80 and 120 mg/dL. Freestyle Libre 3 is similar.
All continuous glucose monitoring manufactures will advise that patients confirm a low (<70 mg/dL) using the fingerstick method. Accuracy of continuous glucose monitors can be compared by examining their MARD score (mean absolute relative difference). The MARD is a statistical measure of CGM accuracy; the lower the number the better.
Because continuous glucose monitors take a glucose reading every 1-5 minutes, they can provide a more accurate picture of glucose control when compared to 1-2 times daily fingersticks or A1c. This newer assessment of glycemic control is called Time in Range.
Will I Still Need to Prescribe a Blood Glucometer?
Yes! —Patients using a continuous glucose monitor (CGM) will still need a traditional glucometer for occasional blood sugar checks. It is important to verify low glucose levels below 70 mg/dL and high levels above 400 mg/dL with a fingerstick to ensure accuracy. Additionally, a glucometer may be necessary during the sensor’s warm-up period or if the CGM data seems inconsistent with symptoms.
Are Continuous Glucose Monitors Safe?
Yes —Continuous glucose monitors are considered safe and have been approved by regulatory bodies around the world, including the US Food and Drug Administration and the European Medicines Agency. Occasionally, skin irritation or infection may occur. However, these risks can be minimized by following the device’s instructions for use and proper site placement.
Are Continuous Glucose Monitors Painful?
No —Continuous glucose monitors are designed to be minimally painful. The process of placing a sensor can be slightly uncomfortable, but it is usually over quickly and should not cause pain. While, some people may experience irritation or discomfort at the site of the sensor, but this can often be alleviated by adjusting the location of the sensor or by using an adhesive patch to secure it in place.
Special Populations
There are special populations to consider when evaluating the appropriateness of prescribing continuous glucose monitoring.
Can a Patient Use a Sensor in Pregnancy?
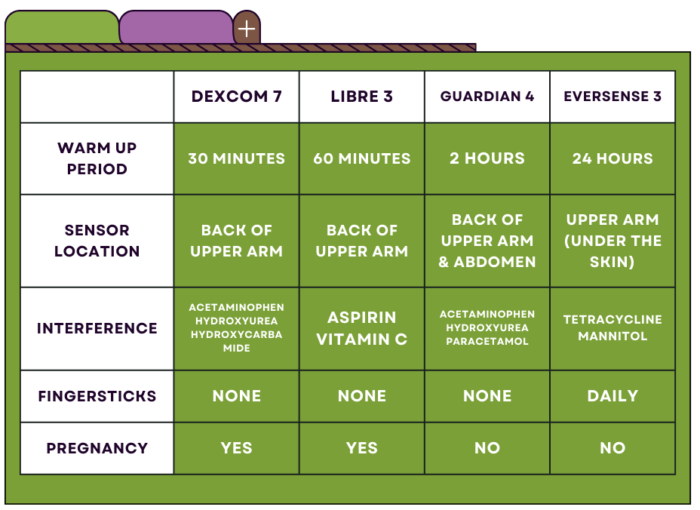
Yes! Both the Dexcom G7 and Libre 3 are approved for use during pregnancy. Continuous glucose monitoring can be incredibly helpful in managing diabetes while pregnant, which can prevent complications for both the mother and baby. Pregnant women with diabetes are at a higher risk of preeclampsia, preterm birth, and birth defects, making tight glucose control essential.
Can a Patient use a Sensor while on Dialysis?
There is no definite answer as to whether a patient can use continuous glucose monitoring while on dialysis. Manufactures will advise against it. In practice—it’s used often.
Typically, a patient on dialysis has impaired kidney function, which may affect the accuracy of CGM readings. The changes in fluid balance during dialysis may also impact glycemic control. Some patients may have limitations that require them to disconnect the device before dialysis for safety reasons.
It is important to consider the benefits and risks of using CGM while on dialysis. Close monitoring of the CGM readings along with traditional blood glucose testing may help avoid hypoglycemic episodes. Minimize hypoglycemia is an import factor when being considered for a kidney transplant.
What About Young Children and Older Adults?
Continuous glucose monitoring devices are frequently used in the pediatric population. Dexcom G7 is approved for children as young as 2 years old. Libre 3 is approved for children as young as 4 years old. Though not approved, continuous glucose monitoring is often off-label in infants (neonates) to optimize glucose control while minimizing risk of hypoglycemia.
While there is no maximum age for continuous glucose monitoring use, advanced age does pose some unique challenges. Adaptation or acceptance of new technologies, insurance coverage, medication interference, and possible visual or motor impairments should be considered.
Family should be included in all new continuous glucose monitoring trainings. Many of the remote-compatible devices allow data sharing of data with parents or caregivers.
Continuous Glucose Monitoring Options
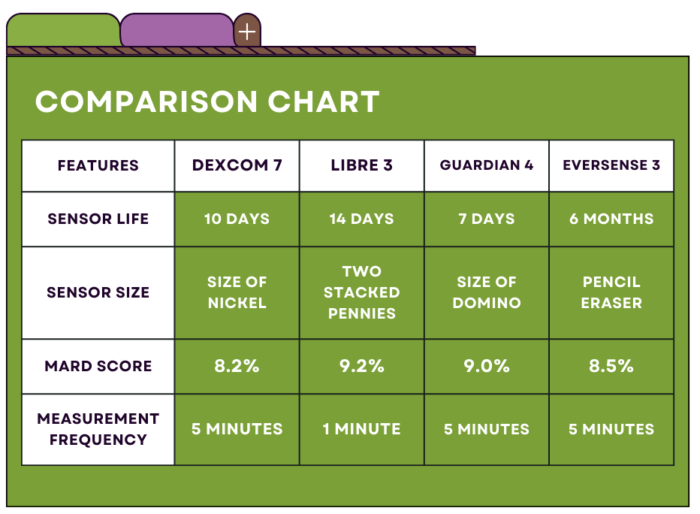
As of November 2023, there are several options available on the US market, including:
1. Dexcom G7 – a smartphone-compatible CGM that alerts users to highs and lows. The older model, Dexcom G6, is integrated with Omnipod 5, Tandem TSlim X2, and Beta Bionics iLet insulin pumps.
2. Freestyle Libre 3 – also smartphone-compatible CGM that alerts users to highs and lows. At a much lower price-point, Libre 3 is a great options for cash-pay users.
3. Medtronic Guardian 4 – a CGM with multiple alarms and customizable alert settings integrated with Medtronic’s 780G insulin pump.
4. Eversense 3 – a CGM that is implanted under the skin and lasts for up to 180 days.
You can view a full comparison of key features across each model in the chart below.
Prescribing a Continuous Glucose Monitor
Are Continuous Glucose Monitors Covered by Commercial Insurance?
Yes —continuous glucose monitors are covered by many commercial insurance plans. Some plans may require prior authorization or have requirements for the type of therapy the patient must be using (i.e., insulin use, number of injections per day, etc.). While most commercial insurances will dispense sensors at the local pharmacy, some plans require the patient to use a mail-order pharmacy or a durable medical equipment (DME) supplier.
Are Continuous Glucose Monitors Covered by Medicare?
Yes —Continuous Glucose Monitors are covered by Medicare for beneficiaries who meet certain criteria. Medicare covers sensors for individuals who meet the following requirements (as of November 2023):
- Must have a diagnosis of diabetes.
- Sufficient training on how to use the prescribed device.
- Prescribed in accordance with its FDA indications for use.
- Must meet at least one of the following criteria;
- Insulin-treated; or,
- A history of problematic hypoglycemia with documentation of recurrent low-events (glucose <54mg/dL) despite multiple attempts to adjust the diabetes treatment plan; or,
- A history of a “level 3” hypoglycemic event, defined as a glucose <54mg/dL with altered mental and/or physical status requiring third-party assistance.
- An in-person or telehealth visit with within the last 6 months with the treating practitioner.
Continuous glucose monitors are considered durable medical equipments and will need to be ordered through a DME-supplier.
Are Continuous Glucose Monitors Covered by Medicaid?
It depends. In Colorado—yes, if the beneficiary meets certain criteria. The Center for Health Care Strategies maintains an updated list of state Medicaid coverage guidelines.
What if Continuous Glucose Monitors are Not Covered?
When continuous glucose monitors are not covered by insurance, patients may choose to pay for the devices themselves. Many of the manufactures offer financial assistance programs.
Key Takeaways
Managing diabetes can be a challenging task, particularly with traditional fingerstick glucometers. However, the rise of continuous glucose monitors has significantly reduced the burden of diabetes self-care.
Continuous glucose monitors are affordable, accessible, relatively pain-free, and very easy to use. Despite some accuracy concerns, continuous glucose monitors provide an incredible volume of data, as well as life-saving alerts and alarms that prevent against dangerous low glucose values.
Managing patients on continuous glucose monitors may feel daunting, but the improvements in quality of life and overall health outcomes far outweigh any administrative burdens.


You May Also Like

Destigmatizing Diabetes
September 3, 2024
Diagnosing Type 1 Diabetes in Adults
August 10, 2022